| Major Groups > True Morels and Verpas |

|
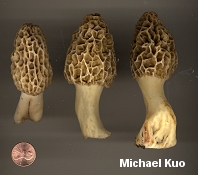
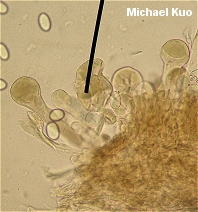
The Morchellaceae: True Morels and Verpas [ Ascomycetes > Pezizales . . . ] by Michael Kuo The Morchellaceae family includes the true morels (members of the genus Morchella), the verpas (in the genus Verpa), and the cup fungi in the genus Disciotis. Under the microscope, these mushrooms all have asci that do not turn blue in iodine, and spores that are smooth, elliptical, and have homogeneous contents. Another defining feature of the family is the large number of nuclei (20-60) found in Morchellaceae spores. Over the last decade or so, visitors to this web site helped sharpen our understanding of the genus Morchella by contributing hundreds of well documented morel collections to the now closed Morel Data Collection Project (MDCP). As a result of study of these collections, along with collections from herbaria and other sources, several papers were published (O'Donnell and collaborators, 2011; Kuo and collaborators, 2012) hypothesizing the evolution of morels and describing 19 DNA-defined species (14 of which were new) in North America. There are undoubtedly more North American morels to be discovered, but the species described by Kuo and collaborators are probably the most commonly encountered; they are keyed out below. Unfortunately the species are not always "morphologically distinct," which means they can't always be identified by looking at their physical features. But when geographic distribution information is added to the picture, only a few frustrating identification dilemmas remain (see couplets 11 and 21 in the key below). Identification characters for Morchella are primarily macroscopic, and involve careful observation of the cap and stem. Microscopic features are informative in a few cases, and include spore size and the morphology of the paraphysis-like elements on the morel's sterile ridges. For a thorough discussion of morphological characters in Morchella see the Supplementary Materials of the Kuo and collaborators publication. As is usually the case with mushroom identification, you will need to have a nice, large collection of fruiting bodies representing both immature and mature stages of development in order to have much success. DNA study of MDCP specimens by Carol Carter and by Kerry O'Donnell exposed several morphological misconceptions about North American morels:
Results reported in O'Donnell and collaborators (2011) and delineated morphologically in Kuo and collaborators (2012) support the idea that the genus Morchella contains three major evolutionary groups, or "clades." The first contains Morchella rufobrunnea only and is therefore labeled the rufobrunnea clade; the second, the esculenta clade, contains 5 species in North America; the final clade, the elata clade, contains 14 North American representatives. Fortunately for traditionalists, the esculenta and elata clades correspond in great part to the concepts of "yellow morels" and "black morels" found in previous treatments--with some exceptions (especially regarding Morchella frustrata and Morchella snyderi). The world still awaits contemporary study of the rest of the Morchellaceae. In the genus Verpa, two morphological species are currently accepted; they are keyed out below, with the morels. However, it would not surprise me at all if results from DNA studies of verpas were to parallel those of the morels, leading to the naming of North American species. The same may be said of the cup fungi in Disciotis. For now, however, Disciotis venosa is the only accepted North American species; I have keyed it in the key to cup fungi. |
© MushroomExpert.Com |
|
Key to Verpas and DNA-Defined True Morels in North America
Insufficiently Known Species, Excluded Species, and Doubtful Names The species below represent invalid names or names that cannot currently be applied to North American morels with scientific accuracy; follow the links for detailed explanations. Morchella atrotomentosa (Moser) Bride; invalid name The names Morchella conica, Morchella crassipes, Morchella deliciosa, Morchella esculenta, Morchella elata, and Morchella semilibera, often used in North American field guides, are European names; due to high continental endemism in Morchella (demonstrated by O'Donnell and collaborators, 2011), these names can safely be discarded as applying to North American morels. References Amir, R., D. Levanon, Y. Hadar & I. Chet (1992). Formation of sclerotia by Morchella esculenta: relationship between media composition and turgor potential in the mycelium. Mycological Research 96: 943-948. Amir, R., D. Levanon, Y. Hadar & I. Chet (1993). Morphology and physiology of Morchella esculenta during sclerotial formation. Mycological Research 97: 683-689. Amir, R., D. Levanon, Y. Hadar & I. Chet (1994). The role of source-sink relationships in translocation during sclerotial formation by Morchella esculenta. Mycological Research 98: 1409-1414. Amir, R., D. Levanon, Y. Hadar & I. Chet (1995). Factors affecting translocation and sclerotial formation in Morchella esculenta. Experimental Mycology 19: 61-70. Amir, R., E. Steudle, D. Levanon, Y. Hadar & I. Chet (1995). Turgor changes in Morchella esculenta during translocation and sclerotial formation. Experimental Mycology 19: 129-136. Apfelbaum, S. I., A. Haney & R. E. Dole (1984). Ascocarp formation by Morchella angusticeps after wildfire. The Michigan botanist 23: 99-102. Azar, I. & Y. Uzun (2017). An interesting half-free morel record for Turkish mycobiota (Morchella populiphila M. Kuo, M. C. Carter & J. D. Moore). Mantar Dergisi / The Journal of Fungus 8: 125–128. Brock, T. D. (1951). Studies on the nutrition of Morchella esculenta Fries. Mycologia 43: 402-422. Bunyard, B. A., M. S. Nicholson & D. J. Royse (1994). A systematic assessment of Morchella using RFLP analysis of the 28s ribosomal RNA gene. Mycologia 86: 762-772. Bunyard, B. A., M. S. Nicholson & D. J. Royse (1995). Phylogenetic resolution of Morchella, Verpa, and Disciotis [Pezizales: Morchellaceae] based on restriction enzyme analysis of the 28S ribosomal RNA gene. Experimental Mycology 19: 223-233. Buscot, F. & Roux, J. (1987). Association between living roots and ascocarps of Morchella rotunda. Transactions of the British Mycological Society 89: 249-252. Buscot, F. (1989). Field observation on growth and development of Morchella rotunda and Mitrophora semilibera in relation to forest soil temperature. Canadian Journal of Botany 67: 589-593. Buscot, F. & Kottke, I. (1990). The association of Morchella rotunda (Pers.) Boudier with roots of Picea abies (L.) Karst. New Phytologist 116: 425-430. Buscot, F. & Bernillon, J. (1991). Mycosporins and related compounds in field and cultured mycelial structures of Morchella esculenta. Mycological Research 95: 752-754. Buscot, F. (1992). Mycorrhizal succession and morel biology. In Mycorrhizas in Ecosystems. Ed. D. J. Read, D. H. Lewis, A. H. Fritter & I. J. Alexander. C.A.B. International. 220-224. Buscot, F. (1992). Synthesis of two types of association between Morchella esculenta and Picea abies under controlled culture conditions. Journal of Plant Physiology 141: 12-17. Buscot, F. (1993). Mycelial differentiation of Morchella esculenta in pure culture. Mycological Research 97: 136-140. Buscot, F., D. Wipf, C. di Battista, J. -C. Munch, B. Botton & F. Martin (1996). DNA polymorphism in morels: PCR/RFLP analysis of the ribosomal DNA spacers and microsatellite-primed PCR. Mycological Research 100: 63-71. Chen, J.-Y. & P.-G. Liu (2005). A new species of Morchella (Pezizales, Ascomycota) from southwestern China. Mycotaxon 93: 89-93. Dahlstrom, J. L., J. E. Smith & N. S. Weber (2000). Mycorrhiza-like interaction by Morchella with species of the Pinaceae in pure culture synthesis. Mycorrhiza 9: 279-285. Dalgleish, H. J. & K. M. Jacobson (2005). A first assessment of genetic variation among Morchella esculenta (morel) populations. Journal of Heredity 96: 396-403. Duchesne, L. C. & Weber, M. G. (1993). High incidence of the edible morel Morchella conica in a jack pine, Pinus banksiana, forest following prescribed burning. The Canadian Field-Naturalist 107: 114-116. Degreef, J., E. Fischer, C. Sharp & O. Raspe (2009). African Morchella crassipes (Ascomycota, Pezizales) revealed by analysis of nrDNA ITS. Nova Hedwigia 88: 11-22. Emery, M. R. & E. S. Barron (2010). Using local ecological knowledge to assess morel decline in the U. S. Mid-Atlantic region. Economic Botany 64: 205-216. Fine, G. A. (1998). Morel tales: The culture of mushrooming. Cambridge: Harvard UP. 324 pp. Fries, E. M. (1822). Morchella, Helvella. Systema Mycologicum, Vol. II: 5-18. Gessner, R. V., M. A. Romano & R. W. Schiltz (1987). Allelic variation and segregation in Morchella deliciosa and M. esculenta. Mycologia 79: 683-687 .Gessner, R. V. (1995). Genetics and systematics of North American populations of Morchella. Canadian Journal of Botany 73 (Suppl. 1): S967-S971. Goldway, M., R. Amir, D. Goldberg, Y. Hadar & D. Levanon (2000). Morchella conica exhibiting a long fruiting season. Mycological Research 104: 1000-1004. Greene, D. F., M. Hesketh & E. Pounden (2010). Emergence of morel (Morchella) and pixie cup (Geopyxis carbonaria) ascocarps in response to intensity of the forest floor combustion during a wildfire. Mycologia 102: 766-773. Grove, J. W. & Hoare, S. C. (1953). The Helvellaceae of the Ottawa district. The Canadian Field-Naturalist 67: 96-102. Guzmán, G. & Tapia, F. (1998). The known morels in Mexico, a description of a new blushing species, Morchella rufobrunnea, and new data on M. guatemalensis. Mycologia 90: 705-714. Harbin, M., & Volk, T. J. (1999). The relationship of Morchella with plant roots. Abstract #1601: XVI International Botanical Congress, St. Louis, Missouri, USA. p. 59. Hervey, A., G. Bistis & I. Leong (1978). Cultural studies of single ascospore isolates of Morchella esculenta. Mycologia 70: 1269-1274. Jacquetant, E. (1984). Les morilles. Paris: La Biblioteque des Arts. 114 pp. Jacquetant, E. (1984). Typifications et mises au point nomenclaturales dans l'ouvrage "LES MORILLES" (de E. JACQUETANT), Nature-Piantanida 1984." Documents Mycologique 14: 1-2. Jung, S. W., R. V. Gessner, K. C. Keudell & M. A. Romano (1993). Systematics of Morchella esculenta complex using enzyme-linked immunosorbent assay. Mycologia 85: 677-684. Kellner, H., Renker, C. & Buscot, F. (2005). Species diversity within the Morchella esculenta group (Ascomycota: Morchellaceae) in Germany and France. Organisms, Diversity & Evolution 5: 101-107. Kellner, H., P. Luis & F. Buscot (2007). Diversity of laccase-like multicopper oxidase genes in Morchellaceae: identification of genes potentially involved in extracellular activities related to plant litter decay. Microbiological Ecology 61: 153-163. Krombholz, J. V. (1834). Naturgereue Abbildungen und Beschreibungen der essbaren, schadlichen und verdachtigen Schwamme. Prague: J. G. Calve. [Morchella (III: 1-16)]. Kuo, M. (2005). Morels. Ann Arbor: University of Michigan Press. 205 pp. Kuo, M. (2008). Morchella tomentosa, a new species from western North America, and notes on M. rufobrunnea. Mycotaxon 105: 441-446. Kuo, M., D. R. Dewsbury, K. O'Donnell, M. C. Carter, S. A. Rehner, J. D. Moore, J.-M. Moncalvo, S. A. Canfield, S. L. Stephenson, A. S. Methven &T. J. Volk (2011). Taxonomic revision of true morels (Morchella) in Canada and the United States. Mycologia 104: 1159-1177. Li, S. H., Y. C. Zhao, H. M. Chai & M. H. Zhong (2006). Two new species in the genus Morchella (Pezizales, Morchellaceae) from China. Mycotaxon 95: 319-322. Linne, C. (Linnaeus). (1753). Phallus. In: Species plantarum: exhibentes plantas rite cognitas ad genera relatas. Tomus II . Page 1178. Linnaeus's Species Plantarum is online here; use the left-hand navigation box to select "Phallus" on page 1178. Linne, C. (Linnaeus). (1767). Phallus. In: Systema Naturae per Regna Tria Naturae, secundum Classes, Ordines, Genera, Species, cum Charicteribus & Differentiis. Tomus II. Page 724. Linnaeus's Systema is online here; use the left-hand navigation box to select "Phallus" on page 724. Masaphy, S. (2005). External ultrastructure of fruit body initiation in Morchella. Mycological Research 109: 504-512. Masaphy, S., L. Zabari & D. Goldberg (2009). New long-season ecotype of Morchella rufobrunnea from northern Israel. Micologia Aplicada International 21: 45-55. McFarlane, E. M., Pilz, D. & Weber, N. S. (2005). High-elevation gray morels and other Morchella species harvested as non-timber forest products in Idaho and Montana. Mycologist 19: 62-68. Mihail, J. D., J. H. Bruhn & P. Bonello (2007). Spatial and temporal patterns of morel fruiting. Mycological Research 111: 339-346. Miller, S. L., P. Torres & T. M. McClean (1994). Persistence of basidiospores and sclerotia of ectomycorrhizal fungi and Morchella in soil. Mycologia 86: 89-95. Moser, M. (1949). Uber das massenauftreten von Formen der Gattung Morchella auf Waldbrandflachen. Sydowia, Annales Mycologici ser. II, 3: 174-195. O'Donnell, K. O., E. Cigelnik, N. S. Weber & J. M. Trappe (1997). Phylogenetic relationships among ascomycetous truffles and the true and false morels inferred from 18S and 28S ribosomal DNA sequence analysis. Mycologia 89: 48-65. O'Donnell, K, A. P. Rooney, G. L. Mills, M. Kuo, N. S. Weber & S. A. Rehner (2011). Phylogeny and historical biogeography of true morels (Morchella) reveals an early Cretaceous origin and high continental endemism and provincialism in the Holarctic. Fungal Genetics and Biology 48: 252-265. Overholts, L. O. (1934). The morels of Pennsylvania. Procedings of the Pennsylvania Academy of Sciences 8:108Ð114. Ower, R. (1982). Notes on the development of the morel ascocarp: Morchella esculenta. Mycologia 74: 142-144. Ower, R. D., G. L. Mills & J. A. Malachowski (1985). United States Patent 4,594,809: Cultivation of Morchella. Retrieved April 7, 2006 from the United States Patent Office Web site. (Available online here.) Pagliaccia, D., G. W. Douhan, L. Douhan, T. L. Peever, L. M. Carris & J. L. Kerrigan (2011). Development of molecular markers and preliminary investigation of the population structure and mating system in one lineage of black morel (Morchella elata) in the Pacific Northwestern USA. Mycologia 103: 969-982. Peck, C. H. (1887). Morchella angusticeps. In: Bulletin of the New York State Museum 2: 21-22. Peck, C. H. (1903). Morchella punctipes. In: New species of fungi. Bulletin of the Torrey Botanical Club 30: 99. Persoon, C. H. (1801). Helvella, Morchella. Synopsis Methodica Fungorum: 614-621. Pilz, D., N. S. Weber, M. C. Carter, C. G. Parks & R. Molina (2004). Productivity and diversity of morel mushrooms in healthy, burned, and insect-damaged forests of northeastern Oregon. Forest Ecology and Management 198: 367-386. Pilz, D., R. McLain, S. Alexander, L. Villareal-Ruiz, S. Berch, T. L. Wurtz, C. G. Parks, E. McFarlane, B. Baker, R. Molina & J. E. Smith (2007). Ecology and management of morels harvested from the forests of western North America. Portland, Oregon: USDA General Technical Report. 161 pp. This publication is available online at the Forest Service: http://www.fs.fed.us/pnw/publications/gtr710/ Richard, F., J. -M. Bellanger, P. Clowez, K. Hansen, K. O'Donnell, A. Urban, M. Sauve, R. Courtecuisse & P. -A. Moreau (2015). True morels (Morchella, Pezizales) of Europe and North America: evolutionary relationships inferred from multilocus data and a unified taxonomy. Mycologia 107: 359–382. Robbins, W. J. & Hervey, A. (1959). Wood extract and growth of Morchella. Mycologia 51: 356-363. Royse, D. J. & May, B. (1990). Interspecific allozyme variation among Morchella spp. and its inferences for systematics within the genus. Biochemical Systematics and Ecology 18: 475-479. Schmidt, E. L. (1979) Puffing in Morchella. Bulletin of the British Mycological Society 13: 126-127. Schmidt, E. L. (1983). Spore germination of and carbohydrate utilization by Morchella esculenta at different soil temperature. Mycologia 75: 870-875. Seaver, F. J. (1928). The North American cup-fungi (operculates). New York: Hafner Publishing Co. 377 p. Singh, S. K., S. Kamal, M. Tiwari, M. C. Yadav & R. C. Upadhyay (2004). Arbitrary primer based RAPD–A useful genetic marker for species identification in morels. Journal of Plant Biochemistry & Biotechnology 13: 7-12. Singh, S. K., M. Tiwari, S. Kamal & M. C. Yadav (2005). Morel phylogeny and diagnostics based on restriction fragment length polymorphism analysis of ITS region of 5.8S ribosomal RNA gene. Journal of Plant Biochemistry and Biotechnology 14: 179-183. Snyder, L. C. (1938). The operculate Discomycetes of western Washington. University of Washington Publications in Biology 8: 1-64. Stefani, F. O. P, S. Sokolski, T. L. Wurtz, Y. Piché, R. C. Hamelin, J. A. Fortin & J. A. Berubé (2010). Morchella tomentosa: A unique belowground structure and a new clade of morels. Mycologia 102: 1082-1088. Taskin H., S. Büyükalaca, H. H. Dogan, S. A. Rehner & K. O'Donnell (2010). A multigene molecular phylogenetic assessment of true morels (Morchella) in Turkey. Fungal Genetics and Biology 47: 672-682. Tiffany, L. H., G. Knaphus & D. M. Huffman (1998). Distribution and ecology of the morels and false morels of Iowa. Journal of the Iowa Academy of Science 105: 1-15. Volk, T. J. & Leonard, T. J. (1989). Experimental studies on the morel. I. Heterokaryon formation between monoascosporous strains of Morchella. Mycologia 81: 523-531. Volk, T. J. & Leonard, T. J. (1990). Cytology of the life-cycle of Morchella. Mycological Research 94: 399-406. Weber, N. S. (1995). A morel hunter's companion: A guide to true and false morels. Michigan: Thunder Bay Press. 209 pp. Winder, R. S. (2006). Cultural studies of Morchella elata. Mycological Research 110: 612-623. Winder, R. S. & M. E. Keefer (2008). Ecology of the 2004 morel harvest in the Rocky Mountain Forest District of British Columbia. Botany 86: 1152-1167. Wipf, D., J. C. Munch, B. Botton & F. Buscot (1996). DNA polymorphism in morels: Complete sequences of the internal transcribed spacer of genes coding for rRNA in Morchella esculenta (yellow morel) and Morchella conica (black morel). Applied and Environmental Microbiology 62: 3541-3543. Wipf, D., S. Koschinsky, P. Clowez, J. C. Munch, B. Botton & F. Buscot (1997). Recent advances in ecology and systematics of morels. Cryptogamie, Mycologie 18: 95-109. Wipf, D., A. Fribourg, J. C. Munch, B. Botton & F. Buscot (1999). Diversity of the internal transcribed spacer of rDNA in morels. Canadian Journal of Microbiology 45: 769-778. Wurz, T. L., A. L. Wiita, N. S. Weber & D. Pilz (2005). Harvesting morels after wildfire in Alaska. USDA: Research Note PNW-RN-546. Yoon, C., R. V. Gessner & M. A. Romano (1990). Population genetics and systematics of the Morchella esculenta complex. Mycologia 82: 227-235. This site contains no information about the edibility or toxicity of mushrooms. Cite this page as: Kuo, M. (2012, November). The Morchellaceae: True morels and verpas. Retrieved from the MushroomExpert.Com Web site: http://www.mushroomexpert.com/morchellaceae.html |