| Major Groups > Gilled Mushrooms > Pale-Spored > Russula |

|
The Genus Russula [ Basidiomycota > Russulales > Russulaceae . . . ] by Michael Kuo The genus Russula includes some very beautiful and interesting species, and a lot of hard-to-distinguish species. Because russulas are typically fairly large, and because they are often brightly colored, amateur mushroomers are frequently interested in identifying them. About 20 or 30 species can be identified fairly easily--but there are perhaps 750 species worldwide. Confounding the identification problems for North American Russula collectors is the dearth of available technical literature. I know several mycologists who maintain that Kauffman's 1918 treatment of Russula species in the Great Lakes area is still one of the most comprehensive and useful overall treatments of the genus on this continent! See the bibliography below if you are interested in attempting to compile a notebook of North American Russula literature by sorting through field guide descriptions and technical treatments of subgenera and sections. Before you do, however, let me try to talk you out of it. Advanced Russula identification is a nightmare far beyond the usual frustrating realm of advanced mushroomology. In fact, I will go ahead and say it (though I am likely to receive some e-flak for my efforts): Russula identification is a joke. The "species" are defined on frequently ridiculous, variable characters; the literature goes to great lengths to cover this fact up with pseudoscientific jargon and long-winded descriptions; the use of a microscope often compounds, rather than alleviates, the frustrating milieu of variability and subjectivity; and we are at a point in time when DNA studies are likely to throw out most of the babies with the PCR-primed bathwater. Since I am an English teacher, I feel obliged to back these points up with evidence in the paragraphs that follow. You don't just call the Emperor naked without proving it. But my main point is that the 20 or 30 species you will find in most field guides to North American mushrooms are about as good as it gets, and I will not be offended if you skip my little tirade and scroll to the links to species pages and keys below. Macroscopic Features The bulk of Russula identification hinges on features you can theoretically determine without using a microscope. Color is among these features, of course, so I will start my tirade by quoting a fairly typical description of the cap color of a red russula: [W]hen young dark grayish red (Dark Livid Brown) to grayish reddish brown (Chocolate) centrally and strong to moderate reddish brown (Burnt Sienna, Hay's Russet, Morocco Red, Brick Red) marginally, or strong yellow (Mustard Yellow) to light yellow (Straw Yellow, Maize Yellow, Cream Color) overall, or moderate orange yellow (Ochraceous-Buff) to strong yellowish brown (Ochraceous-Tawny) centrally and strong to moderate reddish brown marginally, when mature variously colored with grayish reddish brown, strong to moderate reddish brown, strong to light brown (Hazel, Pecan Brown), strong to light yellowish brown (Ochraceous-Tawny, Cinnamon-Buff), dark to moderate reddish orange (Vinaceous-Rufous, Brazil Red, Ferruginous, Rufous), brownish orange (Cinnamon-Rufous), deep to moderate orange (Mars Orange, Orange-Cinnamon), moderate to pale orange yellow (Ochraceous-Buff, Warm Buff), and strong yellow (Mustard Yellow) to pale yellow (Cartridge Buff), usually darker centrally and lighter marginally, but sometimes brownish orange to orange yellow or yellow centrally and darker marginally or even orange yellow to yellow overall. |
|
|
So, Captain Russula, what color was that mushroom? I could spend pages (don't worry; I won't) detailing the logic problems, the slipperiness of the syntax, the faulty parallels, and everything else that is wrong with this passage--but these things are probably immediately obvious to anyone whose life involves coming up for air, out of Russula Land once in a while. The thing is reddish, but sometimes orangish or yellowish, for crying out loud, and the elaborate pseudoscientific rhetoric is an attempt to pin down the un-pin-able with precision. But what the uninitiated may not realize about this passage is that it is written in code--two codes, in fact. The author of the description above (I am leaving him nameless, if you don't mind, because his contributions to mycology are impressive and important and I don't want to poison your impression) indicates elsewhere in this paper that he has used the ISCC-NBS system for color names, along with the Ridgway system. Thus, in the passage quoted, things like "strong yellowish brown" refer to the ISCC-NBS system and the parenthetical, capitalized colors ("Ochraceous-Buff," for example) come from the Ridgway system. If you have ever shopped for paint, you have some experience with stuff like this. The little color strips you brought home and held up to the wall comprise such a system. But if you painted your wall and were disappointed because the color turned out not to be what you thought it was going to be, you have real-world experience with the problems generated by such systems. They should be obvious: people see colors differently, lighting affects perception of color, and so on. None of this deters mycologists, however. Go to a mushroom club's foray, one where professional mycologists are on hand, and you will see them in their field laboratories poring over color charts, held carefully with the mushrooms under special lamps designed to replicate "natural" lighting, jotting down the best color matches in their field notes. Just recently, by the way, mycologists have been circulating a link to the Online Auction Color Chart, which can be purchased for a few dollars, in hopes that communication about color between amateur mushroomers and professional mycologists will be enhanced through reference to a system that is readily available. But let's stop for a minute and discuss the naked Emperor's anatomy. Phallus impudicus arises from a whitish underground egg, and Phallus hadriani arises from a purplish one. Aside from the color difference, there's not a lot separating the two mushrooms. But while this kind of distinction works fairly rigidly in the case of Phallus, it goes totally limp when we get to something like Russula, where radical color differences are often manifested within a single collection of several specimens. And let's not forget that DNA studies have been telling us for several decades now that color differences (in people, animals, plants, and yes, mushrooms) are not particularly indicative of genetic relationships. Even spore print color, in the world of mushrooms, has been shown not to be an indicator of genetic affinity. Speaking of spore prints, let's get back to Russula by recalling that the color of the spore print is an important taxonomical character in defining Russula species. The color of a Russula spore print ranges from white to orangish, and several mini-systems have been established by russulologists to account for degrees of color. Here is one such system (from Kibby & Fatto, 1990) . . .
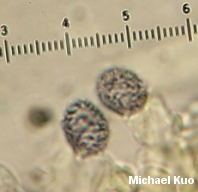
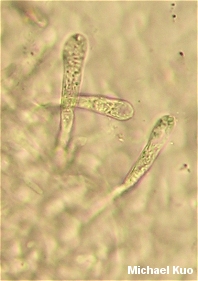
. . . in which descriptions of the species then refer to the corresponding letter for spore print color. The problem is, the color of the spore print in Russula (and in Lactarius, for that matter) can vary substantially. While I have never found a Russula that is supposed to have a pure white spore print manifesting a dark orange one instead, distinctions beyond broad assessments like "pale" and "dark" break down constantly. Throw in the fact that the amount of moisture present in the spore print will affect its color, the fact that spore print color changes over time and is sometimes studied on type specimens dried decades ago, and you have a big mess on your hands. Meanwhile, there are "species" and "varieties" of Russula defined almost entirely on differences of, say, two or three steps in the scale represented above. As long as I am peeling away the Emperor's clothes, I might as well discuss the "peelability" of the cap cuticle in Russula, which is also an important taxonomic feature. When you grab the "skin" of the cap with your thumb and finger and attempt to peel it off the mushroom, what happens? Your answer to this question may help determine what Russula you are looking at, according to Russula literature. The cuticle may be "adnate," adhering tightly to the cap and resisting your efforts. Or it may readily peel away, almost to the center of the cap. Or it may peel one fourth of the way to the center. Or one third. Or halfway. Or two-thirds . . . you get the picture. Perhaps a basic assessment of whether the cuticle adheres tightly or not (say, "peels easily" versus "peels with difficulty") could be fairly universally agreed upon, but I'm telling you now that I can peel the cuticle off of any russula with a little determination, and people's versions of "ease" and "difficulty" are likely to vary substantially. What does the Emperor smell like? For that matter, how does he taste in all his naked glory? You'd better find out, if you're going to identify your Russula--and you'd better be able to navigate fine distinctions between "mild," "slightly acrid," "moderately acrid," "very acrid," and so on, since these distinctions may define your species. And while the Emperor may not blush at his nudity, the stem of your Russula may blush with the (usually reddish, in these cases) color of the cap. If so, you may be looking at one or another species--despite the fact that this feature often varies from collection to collection, or even within a single collection. Microscopic Features Since it is so easily accessed at the moment, we might as well crawl up the Emperor's (5.7 -) 7.5 - 8.5 (- 10) x (3.7 -) 4.5 - 6.5 (- 7.5) . . . where the parenthetical numbers represent rare but recorded measurements. Meanwhile, a completely different Russula has spore measurements only slightly different, varying by something like 1 µ. Russula spores are ornamented with little spines and ridges, so assessing the length of the spines, the frequency and character of the ridges (and so on) became very popular in the middle of the 20th Century, when being a mycologist bordered on being the same thing as a fungal microscopist. This time period is also when much of the most current Russula literature was written, so it should come as no surprise that species are often defined on the basis of spore ornamentation. Are the connecting ridges scattered and isolated? Or do they join up to form broken and partial reticula? If answering these questions sounds easy enough to you, consider this description of the ornamentation on spores belonging to the same mushroom whose colors were described above: Ornamentation of short ridges and cylindric, conic, clavate, or subglobose warts and spines up to 0.7 - 1.2 (-2.0) µ high, these units isolated, aligned, or clustered, or with connectives, the latter variable in number and sometimes attached only to one wart; often forming a partial reticulum or, less commonly, a complete one. Translation: "The spines can be shaped however they want to be shaped and usually measure about 1 µ long, though they are occasionally twice that size; the connecting lines between the spines are usually present and scattered, but may be rare or, on the other extreme, frequent." Perhaps this description would be acceptable if there were no other russulas to compete, defined on the status of the connecting lines ornamenting their spores. But there are, of course, such competitors, and the whole exercise soon becomes meaningless. If you think I'm being too harsh with this assessment, apply logic to the description of the reticulation above. This single description, which acknowledges potential drastic variation in the reticulation of one species, makes it virtually impossible to eliminate the species as an identification candidate for your Russula if you are basing your identification solely on spore ornamentation. Now throw in the fact that variability like this is mentioned for most of the hundreds of russulas described. Other microscopic features are often even less informative than the sporal features. The disposition of cystidia on the gills in Russula is so variable that even the russulologists don't often try to make much of them. The presence or absence of pileocystidia seems to be a fairly good identification character, but the pileocystidia are often extremely variable in their shapes and sizes, even within a single species. Even worse, the technical literature insists on recording the details of pileocystidia that have been mounted in sulphovanillin--an unstable mixture of concentrated sulphuric acid and vanillin crystals that only lasts for an hour or so after being mixed up. (Fortunately the pileocystidia can almost always be successfully seen with a KOH mount.) Redressing the Emperor We should not let an old man stand around naked in the cold. Some day I will have more to say about this, but for now I ask you to imagine that you have spent your life on the cutting edge of science, and advanced our understanding of mushrooms considerably by devoting your time to weary hours of collection and countless days spent looking through a microscope at your finds, carefully recording your discoveries and publishing your findings. Suddenly it is 1980-something, and some kid fresh out of graduate school who wouldn't know a Russula from a bicycle tire in the woods not only tells you that everything you've accomplished in your career was meaningless, but receives all the attention the scientific-academic world has to offer. On the one hand, you can see the writing on the wall and you are certainly enough of a scientist to agree that the ability to read the coded texts in DNA molecules will wind up being the best way for biology to discover how organisms evolved and how they are related to one another. On the other hand, this kid actually appears to believe that his discoveries make any sense whatsoever outside of the context of your life's work. There is the simple fact that the very mushrooms he is testing were collected and named by you and others like you, so he is testing genetic relationships that are meaningless without reference to the morphological variability you spent your life documenting. The fact that Galerina autumnalis and Galerina marginata are the same has no context for meaning outside of the fact that they were initially separated on the basis of their observable features. More generally, the story that this kid and his colleagues will soon be able to tell about the evolution of mushrooms cannot be told coherently as a story about molecular changes alone, since the relationship between these changes and morphology and ecology is the only context in which the kid's story means anything; otherwise, he might just as well calculate pi for the rest of his life and publish each digit with a splash. So How Do I Identify My Russula? Good question. Unless you have collected one of the species traditionally included in field guides you had best buckle your seatbelt and be prepared to ride over the bumpy road of variability in almost every feature you can examine. However, while I have been long-winded in detailing this variability, I hope it has also been clear that 1) there are many features to examine, and 2) variability of features occurs within some limits. Thus, you do sometimes stand a fair chance of getting close with an identification. This chance is increased proportionally to the number of specimens you have to study. If you have picked one red russula and brought it home, you had probably better forget it. On the other hand, if you have made a collection of six or seven specimens representing different stages of development, you will be better prepared to assess what features are variable, and to what extent. And if you have carefully observed your Russula species many times over the years, in different locations, the odds that you'll be able to figure it out are much higher. When I try to identify russulas, I don't even bother if I don't have at least three specimens to work with (unless there is something very distinctive about the mushroom), representing the various stages of maturity. I carefully record all of the ridiculous features--like how far the cap cuticle peels, or whether the spores are "partially" or "completely" reticulate--to the best of my ability, trying to be objective. I have found that it helps to curse loudly while doing this, but this may or may not help you with the process. Then I get out the keys and descriptions, and have at it. I keep my expectations low, because I know that I am likely to end up with three or four possibilities, each of which varies fairly substantially on one or another feature. Identifying russulas is a lot of work, and I wouldn't blame you at all if you decided to simply call every red russula you find "Russula emetica," since that species is found in most field guides. And who knows; maybe we will find out they really are all Russula emetica once their DNA is studied--though it is equally likely we will find out that Russula emetica is the same as some jelly fungus, while there are actually 23 very genetically distinct but morphologically indistinguishable species currently going under the name Russula virescens.*
Russulas at MushroomExpert.Com Shrimp Russulas Blushing & Blackening Russulas Foetid Russulas Russula aeruginea
References Adamčík, S. (2002). Taxonomy of the Russula xerampelina group. Part 2. Taxonomic and nomenclatural study of Russula xerampelina and R. erythropoda. Mycotaxon 82: 241–267. Adamčik, S. & B. Buyck (2010). Re-instatement of Russula levyana Murrill as a good and distinct American species of Russula section Xerampelinae. Cryptogamie, Mycologie 31: 119–135. Adamčik, S., D. Mitchell & B. Buyck (2010). Russula ochrifloridana sp. nov., a new yellowish fishy Russula from Florida and its comparison with R. grundii. Cryptogamie, Mycologie 31: 363–372. Adamčik, S. & B. Buyck (2011). The species of Russula subsection Xerampelinae described by C. H. Peck and Miss G. S. Burlingham. Cryptogamie, Mycologie 32: 63–81. Adamčík, S. & B. Buyck (2014). Type studies in Russula subsection Nigricantes from the eastern United States. Cryptogamie, Mycologie 35: 293–309. Adamčik, S. & 26 coauthors (2019). The quest for a globally comprehensible Russula language. Fungal Diversity 99: 369–349. Avis, P. G., D. J. McLaughlin, B. C. Dentinger & P. B. Reich (2003). Long-term increase in nitrogen supply alters above- and below-ground ectomycorrhizal communities and increases the dominance of Russula spp. in a temperate oak savanna. New Phytologist 160: 239–253. Bazzicalupo, A. L., B. Buyck, I. Saar, J. Vauras, D. Carmen & M. L. Berbee (2017). Troubles with mycorrhizal mushroom identification where morphological differentiation lags behind barcode sequence divergence. Taxon 66: 791–810. Beardslee, H. C. (1918). The russulas of North Carolina. Elisha Mitchell Scientific Society 33: 147–197. Bergemann, S. E. & S. L. Miller (2002). Size, distribution, and persistence of genets in local populations of the late-stage ectomycorrhizal basidiomycete, Russula brevipes. New Phytologist 156: 313–320. Bergemann, S. E., G. W. Douhan, M. Garbelotto & S. L. Miller (2006). No evidence of population structure across three isolated subpopulations of Russula brevipes in an oak/pine woodland. New Phytologist 170: 177–184. Bills, G. F. & Miller, O. K. Jr (1984). Southern Appalachian Russulas. I. Mycologia 76: 975–1002. Bills, G. F. (1984). Southern Appalachian russulas II. Mycotaxon 21: 491–517. Bills, G. F. (1985). Southern Appalachian russulas. III. The identity of Russula eccentrica and R. morgani (Russulaceae). Brittonia 37: 360–365. Bills, G. F. (1989). Southern Appalachian russulas IV. Mycologia 81: 57–65. Burlingham, G. S. (1915). Russula. North American Flora 9: 201–236. Burlingham, G. S. (1918). New species of Russula from Massachusetts. Mycologia 10: 93–96. Burlingham, G. S. (1924). Notes on species of Russula. Mycologia 16: 16–23. Burlingham, G. S. (1936). New or noteworthy species of Russula and Lactaria. Mycologia 28: 253–267. Buyck, B. (1991). The study of microscopic features in Russula 1. spores and basidia. Russulales News 1: 8–26. Buyck, B. (1991). The study of microscopic features in Russula 2. sterile cells of the hymenium. Russulales News 1(2): 62–85. Buyck, B. (1995). Towards a global and integrated approach on the taxonomy of Russulales. Russulales News 3: 3–17. Buyck, B. & E. Horak (1999). New taxa of pleurotoid Russulaceae. (1999). Mycologia 91: 532–537. Buyck, B. & C. L. Ovrebo (2002). New and interesting Russula species from Panama. Mycologia 94: 888–901. Buyck, B. & D. Mitchell (2003). Russula lentiginosa sp. nov. from West Virginia, USA: a probable link between tropical and temperate Russula-groups. Cryptogamie, Mycologie 24: 317–325. Buyck, B. (2004). Short diagnoses and descriptions for some exotic Russula (Basidiomycotina). Cryptogamie, Mycologie 25: 127–128. Buyck, B. (2006). Provisional key to subsection Virescentinae in the U. S. Retrieved March, 2009 from the Russulales News Web site: http://www.mtsn.tn.it/russulales-news/id_virescentinae.asp Buyck, B.,D. Mitchell & J. Parrent (2006). Russula parvovirescens sp. nov., a common but ignored species in the eastern United States. Mycologia 98: 612–615. Buyck, B., S. Adamcik & D. P. Lewis (2008). Russula section Xerampelinae in Texas. Cryptogamie, Mycologie 29: 121–128. Buyck, B., V. Hofstetter, U. Eberhardt, A. Verbeken & F. Kauff (2008). Walking the thin line between Russula and Lactarius: the dilemma of Russula subsect. Ochricompactae. Fungal Diversity 28: 15–40. Buyck, B. & S. Adamčik (2013). The Russula xerampelina complex (Russulales, Agaricomycotina) in North America. Scripta Botanica Belgica 51: 117–131. Fatto, R. M. (1996). Russula subalbidula - A southern species. Mycotaxon 59: 33–35. Fatto, R. M. (1998). A study of selected Murrill's russulas. Mycotaxon 69: 487–502. Fatto, R. M. (1999). Three new species of Russula. Mycotaxon 70: 167–175. Fatto, R. M. (2000). Several russulas of the Chiricahua mountains. Mycotaxon 75: 265–272. Fatto, R. M. (2002). Some russulas of the section Urentinae. Mycotaxon 84: 229–244. Hesler, L. R. (1960). A study of Russula types. Memoirs of the Torrey Botanical Club 21. 59 pp. Hesler, L. R. (1961). A study of Russula types, II. Mycologia 53: 605–625. Hesler, L. R. (1961). A study of Julius Schaeffer's russulas. Lloydia 24: 182–198. Homola, R. L. (1975). A new russula of the subsection Nigricantes from northeastern North America. Mycologia 67: 428–434. Kauffman, C. H. (1909). Unreported fungi for 1908, with a monograph of the Russulas of the state. Report of the Michigan Academy of Science 11: 55–91. Kibby, G. & Fatto, R. (1990). Keys to the species of Russula in northeastern North America. Somerville, NJ: Kibby-Fatto Enterprises. 70 pp. Knudsen, H., J. Ruotsalainen & J. Vauras (2018). Russula Pers. In Knudsen, H. & J. Vesterholt, eds. (2008). Funga Nordica: Agaricoid, boletoid and cyphelloid genera. Copenhagen: Nordsvamp. 144–186. Kränzlin, F. (2005). Fungi of Switzerland: A contribution to the knowledge of the fungal flora of Switzerland. Volume 6 Russulaceae. Transl. Walters, V. L. & Walters, J. F. Lucern: Verlag Mykologia. 317 pp. Larsson, E. & K. H. Larsson (2003). Phylogenetic relationships of russuloid basidiomycetes with an emphasis on aphyllophoralean taxa. Mycologia 95: 1037–1065. Miller, S. L., T. M. McClean, J. F. Walker & B. Buyck (2001). A molecular phylogeny of the Russulales including agaricoid, gasteroid and pleurotoid taxa. Mycologia 93: 344–354. Miller, S. L. (2004). New and interesting species of Russula from the southeastern United States 1. Russula billsii. Mycotaxon 89: 31–38. Miller, S. L., E. Larsson, K. H. Larsson, A. Verbeken & J. Nuytinck (2006). Perspectives in the new Russulales. Mycologia 98: 960–970. Noffsinger, C. & C. L. Cripps (2021). Systematic analysis of Russula in the North American Rocky Mountain alpine zone. Mycologia 113: 1278–1315. Noffsinger, C. R. & 12 coauthors (2024). Three new species in Russula subsection Xerampelinae supported by genealogical and phenotypic coherence. Mycologia 116: 322–349. Pearson, A. A. (1950). The genus Russula. 2nd. Edition. London: Brown & Sons. 24 p. Peck, C. H. (1906). New York species of Russula. Report of the state botanist. New York State Museum Bulletin 116: 67–117. Redecker, D. T. M. Szaro, R. J. Bowman & T. D. Bruns (2001). Small genets of Lactarius xanthogalactus, Russula cremoricolor, and Amanita franchetii in late-stage ectomycorrhizal successions. Molecular Ecology 10: 1025–1034. Roberts, C. (2007). Russulas of southern Vancouver Island coastal forests. Doctoral dissertation, University of Victoria. Victoria BC, Canada. Shaffer, R. L. (1962). The subsection Compactae of Russula. Brittonia 14: 254–284. Shaffer, R. L. (1964). The subsection Lactarioideae of Russula. Mycologia 56: 202-231. Shaffer, R. L. (1970). Notes on subsection Crassotunicatineae of Russula. Lloydia 33: 49–96. Shaffer, R. L. (1972). North American Russulas of the subsection Foetentinae. Mycologia 64: 1008–1053. Shaffer, R. L. (1975). Some common North American species of Russula Subsect. Emeticinae. Nova Hedwigia 51: 207–237. Shaffer, R. L. ( 1989). Four white-capped species of Russula (Russulaceae). Memoirs of the New York Botanical Garden 49: 348–354. Shaffer, R. L. (1990). Notes on the Archaeinae and other russulas. Contributions to the University of Michigan Herbarium 17: 295–306. Simpson, W. Synonymy of North American Russula. A literature survey. Russulales News 5: 23–33. Thiers, H. D. (1997a). New species of Russula from California. Mycotaxon 63: 349–358. Thiers, H. D. (1997b). The Agaricales (gilled fungi) of California 9. Russulaceae I. Russula. Eureka, CA: Mad River P. Woo, B. (1989). Trial field key to the species of Russula in the Pacific Northwest. Retrieved March, 2009 from the Pacific Northwest Key Council Web site: http://www.svims.ca/council/Russul.htm This site contains no information about the edibility or toxicity of mushrooms. Cite this page as: Kuo, M. (2009, March). The genus Russula. Retrieved from the MushroomExpert.Com Web site: http://www.mushroomexpert.com/russula.html © MushroomExpert.Com |