| Major Groups > Boletes > Leccinum |

|
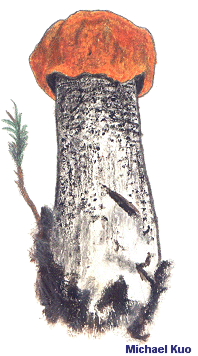
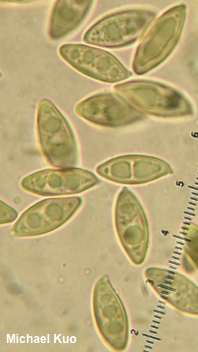
The Genus Leccinum and Leccinoid Fungi [ Basidiomycota > Boletales > Boletaceae . . . ] by Michael Kuo The genus Leccinum has recently been broadly defined (Kuo & Ortiz-Santana 2020) in order to reflect how the mushrooms have evolved together, so there are now two distinct groups of mushrooms in the genus. The first group consists of underground, truffle-like fungi (for example Leccinum caespitosum) that were placed until recently in the genera Chamonixia, Octaviania, Rossbeevera, and Turmalinea. The second group, the one my friend Hoa calls the "sexy Lexies," contains boletes; the mushrooms are soft-fleshed mycorrhizal partners with trees, featuring tubes and pores on the underside of a cap that sits atop a central stem. Unlike most other boletes, however, the stems of leccinoid mushrooms are punctuated with scabers, which typically become brown or black by the time the mushroom is mature—though in a few species the scabers are light in color, reddish, or nearly invisible to the naked eye. (If you are new to bolete identification, compare scabers carefully with glandular dots and reticulation). Aside from the scabrous stems, there is something particularly Leccinum-esque about leccinoid mushrooms; their stature and colors are somehow distinctive. But while recognizing that a bolete is a Leccinum is usually relatively easy, figuring out what species you have found can be truly frustrating. In fact, if you are a North American collector at this point in time, it may not be possible to identify many Leccinum species with scientific confidence. One thing that is clear, however, is that Leccinum species are "host-specific," which means that they only associate with certain kinds of trees. Some species and groups of species appear to be pickier than others, but virtually none qualifies as an all-out "generalist," capable of associating with just about any tree. This means that would-be Leccinum identifiers will need to pay attention to the trees in the vicinity of their collections, because this information is likely to be essential in identifying the mushrooms. Physical features that come into play with Leccinum identification include the color of the cap, as well as the color of the pore surface and whether it changes color when bruised. Color changes of the flesh, when sliced open, should also be noted—and these sometimes take quite a little while to manifest, so I recommend slicing a Leccinum in half lengthwise as you begin the process of taking notes, then observing it every five or ten minutes for at least half an hour. The margin of the cap, in many species, features a sterile overhanging portion that splits as the mushroom grows, creating little flaps of tissue; in other leccinoid mushrooms these flaps are missing. The color of the stem's scabers is important, ranging from nearly white to brown or black—and sometimes the color changes as the mushroom matures, so you will need both young and old specimens to assess this feature confidently. Microscopic examination can provide useful data for Leccinum identification. The morphology of spores, hymenial cystidia, caulocystidia, and the pileipellis should all be recorded, using 2% KOH as a mounting medium. In the 1960s and 70s there was some interest in tissue reactions to Melzer's reagent, but these putatively important reactions have since been shown to be inconsistent and ultimately uninformative. Pages for Leccinoid Fungi Harrya chromapes |
|
|
References Binder, M. & H. Besl (2000). 28S rDNA sequence data and chemotaxonomical analyses on the generic concept of Leccinum (Boletales). In: Associazione Micologica Bresadola, ed. Micologia 2000. Brescia, Italy: Grafica Sette, 75–86. Both, E. E. (1993). The boletes of North America: A compendium. Buffalo NY: Buffalo Museum of Science. 436 pp. Den Bakker, H. C., B. Gravendeel & T. W. Kuyper (2004a). An ITS phylogeny of Leccinum and an analysis of the evolution of minisatellite-like sequences within ITS1. Mycologia 96: 102–118. Den Bakker, H. C., G. C. Zuccarello, T. W. Kuyper & M. E. Noordeloos (2004b). Evolution and host specificity in the ectomycorrhizal genus Leccinum. New Phytologist 163: 201–215. Den Bakker, H. C. & M. E. Noordeloos (2005). A revision of European species of Leccinum Gray and notes on extralimital species. Persoonia 18: 511–587. Den Baker, H. C., G. C. Zuccarello, Th. W. Kuyper & M. E. Noordeloos (2007). Phylogeographic patterns in Leccinum sect. Scabra and the status of the arctic-alpine species L. rotundifoliae. Mycological Research 111: 663–672. Grund, D. W. & Harrison, K. A. (1976). Nova Scotian Boletes. Germany: J. Cramer. 283 pp. Halling, R. E. (1999). New Leccinums from Costa Rica. Kew Bulletin 54: 747–753. Halling, R. E. & G. M. Mueller (2003). Leccinum (Boletaceae) in Costa Rica. Mycologia 95: 488–499. Halling, R. E., M. Nuhn, T. Osmundson, N. Fechner, J. M. Trappe, K. Soytong, D. Arora, D. S. Hibbett & M. Binder (2012). Affinities of the Boletus chromapes group to Royoungia and the description of two new genera, Harrya and Australopilus. Australian Systematic Botany 25: 418–431. Halling, R. E., N. Fechner, M. Nuhn, T. Osmundson, K. Soytong, D. Arora, M. Binder & D. Hibbett (2015). Evolutionary relationships of Heimioporus and Boletellus (Boletales), with an emphasis on Australian taxa including new species and new combinations in Aureoboletus, Hemileccinum and Xerocomus. Australian Systematic Botany 28: 1–22. Kuo, M. & B. Ortiz-Santana (2020). Revision of leccinoid fungi, with emphasis on North American taxa, based on molecular and morphological data. Mycologia 112: 197–211. Lannoy, G. & A. Estades (1995). Monographie des Leccinum d’Europe. La Roche-sur-Foron, France: Federation Mycologique Dauphine-Savoie. 229 pp. Martin, F., J. Diez, B. Dell & C. Delaruelle (2002). Phylogeography of the extomycorrhizal Pisolithus species as inferred from nuclear ribosomal DNA ITS sequences. New Phytologist 153: 345–357. Noordeloos, M. E. & H. C. den Bakker (2018). Leccinum S. F. Gray. In Noordeloos, M. E., T. W. Kuyper, I. Somhorst & E. C. Vellinga, eds. Flora Agaricina Neerlandica: Critical revisions of families of agarics and boleti occurring in the Netherlands. Volume 7. Orrigio, Italy: Candusso Editrice, 163–185. Ortiz-Santana, B., D. J. Lodge, T. J. Baroni & E. E. Both (2007). Boletes from Belize and the Dominican Republic. Fungal Diversity 27: 247–416. Redhead, S. A. & R. Watling (1979). A new psammophilic Leccinum. Canadian Journal of Botany 57: 117–119. Selosse, M. A. (2003). Founder effect in a young Leccinum duriusculum (Schultzer) Singer population. Mycorrhiza 13: 143–149. Singer, R. (1947). The Boletinae of Florida with notes on extralimital species. III. The American Midland Naturalist 37: 1–263. Singer, R. & R. Williams (1992). Some boletes from Florida. Mycologia 84: 724–728. Smith, A. H. & R. Singer (1959). Studies on secotiaceous fungi—IV: Gastroboletus, Truncocolumella, and Chamonixia. Brittonia 11: 205–223. Smith, A. H., H. D. Thiers & R. Watling (1966). A preliminary account of the North American species of Leccinum, Section Leccinum. The Michigan Botanist 5: 131–178. Smith, A. H., H. D. Thiers & R. Watling (1967). A preliminary account of the North American species of Leccinum, Sections Luteoscabra and Scabra. The Michigan Botanist 6: 107–154. Smith, A. H., H. D. Thiers & R. Watling (1968). Notes on species of Leccinum. I. Additions to Section Leccinum. Lloydia 31: 252–267. Smith, A. H. & H. D. Thiers (1971). The boletes of Michigan. Ann Arbor: U Michigan P. 428 pp. Snell, W. H., A. H. Smith & L. R. Hesler (1940). New species of boleti from Cades Cove in the Great Smokies. Journal of the Elisa Mitchell Society 56: 325–328. Snell, W. H. (1942). New proposals relating to the genera of Boletaceae. Mycologia 34: 403–411. Snell, W. H. & Dick, E. A. (1970). The boleti of northeastern North America. Germany: J. Cramer. 115 pp. Šutara, J. (1989). The delimitation of the genus Leccinum. Česká Mykologie 28: 129–137. Thiers, H. D. (1971). California boletes. IV. The genus Leccinum. Mycologia 63: 261–276. Thiers, H. D. (1976). Boletes of the southwestern United States. Mycotaxon 3: 261–273. Wells, V. L. & P. E. Kempton (1967). Studies on the fleshy fungi of Alaska. I. Lloydia 30: 258–268. Wells, V. L. & P. E. Kempton (1975). New and interesting fungi from Alaska. Nova Hedwigia 51: 347–358. Wu, G., B. Feng, J. Xu, X.-T. Zhu, Y.-C. Li, N.-K. Zeng, Md. I. Hosen & Z. L. Yang (2014), Molecular phylogenetic analyses redefine seven major clades and reveal 22 new generic clades in the fungal family Boletaceae. Fungal Diversity 69: 93–115. This site contains no information about the edibility or toxicity of mushrooms. Cite this page as: Kuo, M. (2020, January). The genus Leccinum and leccinoid fungi. Retrieved from the MushroomExpert.Com Web site: http://www.mushroomexpert.com/leccinum.html © MushroomExpert.Com |