| Major Groups > Polypores |

|
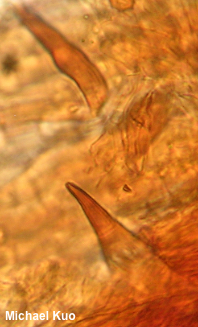
[ Basidiomycota . . . ] The Polypores by Michael Kuo, 2 October 2022 The polypores form a large group of diverse mushrooms. Most of these are wood decomposers whose spore-making machinery is set up within tubes whose ends make up a surface of many pores ("poly-pores")—rather like the tubes of the boletes, except that with some exceptions the tube layer of a polypore cannot be easily removed as a layer, the way it often can with a bolete. Also, the caps of polypores tend not to be round like bolete caps, polypores usually lack central stems, and the flesh of polypores is usually tougher (though there are exceptions for everything in the list). Polypores are saprobes that assist in the decomposition of deadwood—and, in some cases, they are pathogenic, acting as parasites and slowly killing their hosts. The mycelium of polypores consumes the wood differently, depending on the species; some consume lignin and create a white rot, while others consume cellulose, making them brown rot fungi. Taxonomically, the polypores are complicated, and still not completely understood. Seventy years ago, when L. O. Overholts' thorough study of polypores in North America (1953) was published, most of the species of polypores went under the genus name Polyporus. Today, Polyporus is a comparatively small genus, the polypores are distributed among many genera, and DNA studies shift the polypores around on what seems like a daily basis. Identification of polypores ranges from easy to very difficult. Careful analysis of the mushroom's macrofeatures is sometimes sufficient to reach a reasonably secure identification decision. The pore surface of a polypore is sometimes distinctive; for example, Daedaleopsis confragosa has a maze-like pore surface, easily distinguished from the pore surfaces of the many polypores with tiny, round pores. Be sure to pay careful attention to the "host" of your polypore, since identification can sometimes hinge on this information. When polypores grow on living trees, this is a matter of identifying the tree. But polypores are often found on deadwood—in which case one must try to figure out what kind of deadwood is involved. Notice, at the very least, whether your mushroom grows in conifer woods or among hardwoods. However, if your mushroom is on a very large stump in the midst of a forest full of small trees, it may take some research or guesswork to determine what kind of tree the stump may represent. Other features often important in the identification of polypores include the reaction of the flesh to KOH, and microscopic features . . . and here the ugly truth must be told. Microscope work with polypores is a pain in the ass. A bonafide shit show. Imagine unpleasant hours spent soaking and re-soaking mounts, cracking coverslips again and again, trying to slice paper-thin sections out of material with the consistency of a rock, yelling, bleeding, re-soaking again, cracking more coverslips, then going downstairs to have dinner with your family with eyes bulging and smoke pouring out of your ears. And after all of that, the odds are high that you will not have seen the spores, since these freaking mushrooms sit out in the woods for months emptying themselves. As for the hyphal system, which should be "monomitic," "dimitic," or "trimitic," you will ultimately wind up declaring it maybemitic, because what you have actually seen under the microscope is a dark brown mass of whatever-the-hell-that-is, with a few hyphal ends sticking out—ends which you can't focus on because the mount is too thick. Are there setae in the hymenium? Answering this question presupposes one's ability to find the hymenium (good luck), and to cut a section thin enough to isolate the setae from all the rest of the dark brown tissues in the dark brown mass. In short, maybe you should do your family a solid and leave polypore microscopy to the masochists who make careers out of it.
|
|
|
Incomplete Key to North American Polypores I have not yet completed a key to North American polypores, but I have started the ball rolling with a key to the pale-fleshed, stemmed polypores, and keys to the genera Ganoderma and Laetiporus.
Polypore Pages Abortiporus biennis References Al-Mughrabi, K. & T. Hsiang (1998). The mating system of Daedaliopsis confragosa. Mycologia 90: 82–84. Baldrian, P. & Gabriel, J. (2002). Intraspecific variability in growth response to cadmium of the wood-rotting fungus Piptoporus betulinus. Mycologia 94: 428–436. Binder, M., D. S. Hibbett, K. -H. Larsson, E. Larsson, E. Langer & G. Langer (2005). The phylogenetic distribution of resupinate forms across the major clades of mushroom-forming fungi (Homobasidiomycetes). Systematics and Biodiversity 3: 1–45. Binder, M., A. Justo, R. Riley, A. Salamov, F. Lopez-Giraldez, E. Sjökvist, A. Copelan, B. Foster, H. Sun, E. Larsson, K.-H. Larsson, J. Townsend, I. V. Grigoriev & D. S. Hibbett (2017). Phylogenetic and phylogenomic overview of the Polyporales. Mycologia 105: 1350–1373. Blackwell, M. & R. L. Gilbertson (1985). A new species of Inonotus (Aphyllophorales, Hymenochaetaceae) on oak in Louisiana. Mycotaxon 23: 285–290. Brodie, H. J. (1951). The function of the cups of Polyporus conchifer. Science 114: 636. Brusis, O. A. (1972). A new species of Fistulina from Mexico. Mycologia 64: 1248–1251. Burdsall, H. H. (1971). Notes on some lignicolous Basidiomycetes of the southeastern United States. Journal of the Elisha Mitchell Scientific Society 87: 239–245. Burdsall, H. H. Jr. & Banik, M. T. (2001). The genus Laetiporus in North America. Harvard Papers in Botany 6: 43–55. Calonge, F. D., A. García, M. Sanz & J. Bastardo (2003). Buglossoporus quercinus (Basidiomycotina, Coriolaceae), nueva cita para España. Boletín de la Sociedad Micológica de Madrid 27: 33–35. Canfield, E. R. & R. L. Gilbertson (1971). Notes on the genus Albatrellus in Arizona. Mycologia 63: 964–971. Carlson, A., A. Justo & D. S. Hibbett (2014). Species delimitation in Trametes: a comparison of ITS, RPB1, RPB2 and TEF1 gene phylogenies. Mycologia 106: 735–745. Carranza-Morse, J. & R. L. Gilbertson (1986). Taxonomy of the Fomitopsis rosea complex (Aphyllophorales; Polyporaceae). Mycotaxon 25: 469–486. Chen, J., B. Cui, S. He, J. A. Cooper, M. D. Barrett, J. Chen, J. Song & Y. Dai (2016). Molecular phylogeny and global diversity of the remarkable genus Bondarzewia (Basidiomycota, Russulales). Mycologia 108: 697–708. Crockatt, M. (2008). Ecology of the rare oak polypore Piptoporus quercinus and the tooth fungi Hericium cirrhatum, H. coralloides, and H. erinaceus in the UK. Doctor of Philosophy thesis, Cardiff University. 115 pp. Cui, B. -K., J. Vlasák & Y. -C. Dai (2014). The phylogenetic position of Osteina obducta (Polyporales, Basidiomycota) based on samples from northern hemisphere. Chiang Mai Journal of Science 41: 838–845. Dai, Y.-C., H.-J. Xue, J. Vlasak, M. Rajchenberg, B. Wang & L.-W. Zhou (2014). Phylogeny and global diversity of Polyporus group Melanopus (Polyporales, Basidiomycota). Fungal Diversity 64: 133–144. Das, K., A. Parihar & M. E. Hembrom (2015). A new species of Bondarzewia from India. Turkish Journal of Botany 39: 128–133. David, A. & M. Rajchenberg (1985). Pore fungi from French Antilles and Guiana. Mycotaxon 22: 285–325. De, A. B. (1996). Royoporus—a new genus for Favolus spathulatus. Mycotaxon 60: 143–148. De, A. B. (1997). Taxonomy of Royoporus badius comb. nov. Mycotaxon 65: 469–474. De, A. B. (1998). Taxonomy of Royoporus pseudobetulinus comb. nov. Mycotaxon 69: 137–143. Decock, C. (2007). On the genus Microporellus, with two new species and one recombination (M. papuensis spec. nov., M. adextrinoideus spec. nov., and M. terrestris comb. nov.). Czech Mycology 59: 153–170. Fischer, M. & M. Binder (2004). Species recognition, geographic distribution and host-pathogen relationships: a case study in a group of lignicolous basidiomycetes, Phellinus s.l. Mycologia 96: 799–811. Gilbertson, R. L. & Burdsall, H. H. Jr. (1972). Phellinus torulosus in North America. Mycologia 64: 1258–1269. Gilbertson, R. L. (1976). The genus Inonotus (Aphyllophorales: Hymenochaetaceae) in Arizona. Memoirs of the New York Botanical Garden 28: 67–85. Gilbertson, R. L. (1980). Wood-rotting fungi of North America. Mycologia 72: 1–49. Gilbertson, R. L. & Ryvarden, L. (1986). North American polypores. Vol. 1. Oslo: Fungiflora. Gilbertson, R. L. & Ryvarden, L. (1987). North American polypores. Vol. 2. Oslo: Fungiflora. Ginns, J. (2017). Polypores of British Columbia (Fungi: Basidiomycota). Province of British Columbia, Victoria, British Columbia. Technical Report 104. Goldstein, D. & R. L. Gilbertson (1981). Cultural morphology and sexuality of Inonotus arizonicus. Mycologia 73: 167–180. Gottlieb, A. M., J. E. Wright & J.-M. Moncalvo (2002). Inonotus s. l. in Argentina--morphology, cultural characters and molecular analyses. Mycological Progress 1: 229–313. Grand, L. F. & Vernia, C. S. (2004). Biogeography and hosts of poroid wood decay fungi in North Carolina: species of Phellinus and Schizopora. Mycotaxon 89: 181–184. Haight, J. -E., G. A. Laursen, J. A. Glaeser & D. L. Taylor (2016). Phylogeny of Fomitopsis pinicola: a species complex. Mycologia 108: 925–938. Haight, J. -E., K. K. Nakasone, G. A. Laursen, S. A. Redhead, D. L. Taylor & J. A. Glaeser (2019). Fomitopsis mounceae and F. schrenkii—two new species from North America in the F. pinicola complex. Mycologia 111: 339–357. Han, M. -L., Y. -Y. Chen, L. -L. Shen, J. Song, J. Vlasák, Y. -C. Dai & B. -K. Cui (2016). Taxonomy and phylogeny of the brown-rot fungi: Fomitopsis and its related genera. Fungal Diversity 80: 343–373. Harrington, T. C. (1980). Release of airborne basidiospores from the pouch fungus, Cryptoporus volvatus. Mycologia 72: 926–936. Hibbett, D. S. & Donoghue, M. J. (1994). Progress toward a phylogenetic classification of the Polyporaceae through parsimony analysis of mitochondrial ribosomal DNA sequences. Canadian Journal of Botany 73: S853 Hong, S. G. & H. S. Jung (2004) Phylogenetic analysis of Ganoderma based on nearly complete mitochondrial small-subunit ribosomal DNA sequences. Mycologia 96: 742–755. Ji, X. -H., S. -H. He, J. -J. Chen, J. Si, F. Wu, L. -W. Zhou, J. Vlasák, X. -M. Tian & Y. -C. Dai (2017). Global diversity and phylogeny of Onnia (Hymenochaetaceae) species on gymnosperms. Mycologia 109: 27–34. Ji, X., J. L. Zhou, C. G. Song, T. M. Xu, D. M. Wu & B. K. Cui (2022). Taxonomy, phylogeny and divergences times of Polyporus (Basidiomycota) and related genera. Mycosphere 13: 1–52. Justo, A. & D. S. Hibbett (2011). Phylogenetic classification of Trametes (Basidiomycota, Polyporales) based on a five-marker dataset. Taxon 60: 1567–1583. Justo, A., O. Miettinen, D. Floudas, B. Ortiz-Santana, E. Skökvist, D. Lindner, K. Nakasone, T. Niemelä, K.-H. Larsson, L. Ryvarden & D. S. Hibbett (2017). A revised family-level classification of the Polyporales (Basidiomycota). Fungal Biology 121: 798–824. Kim, K. M., J. S. Lee & H. S. Jung (2007). Fomitopsis incarnatus sp. nov. based on generic evaluation of Fomitopsis and Rhodofomes. Mycologia 99: 833–841. Ko, K. S., S. G. Hong & H. S. Jung (1997). Phylogenetic analysis of Trichaptum based on nuclear 18S, 5.8S and ITS ribosomal DNA sequences. Mycologia 89: 727–734. Ko, K. S., H. S. Jung & L. Ryvarden (2001). Phylogenetic relationships of Hapalopilus and related genera inferred from mitochondrial small subunit ribosomal DNA sequences. Mycologia 93: 270–276. Kotiranta, H., V. A. Mukhin, N. Ushakova & Y. -C. Dai (2005). Polypore (Aphyllophorales, Basidiomycetes) studies in Russia. 1. South Ural. Annales Botanici Fennici 42: 427–451. Kout, J. & J. Vlasak (2007). Trametes gibbosa (Basidiomycetes, Polyporales) in the USA and Canada. Canadian Journal of Botany 85: 342–346. Kruger, D. (2002). Monographic studies in the genus Polyporus (Basidiomycotina). Ph. D. thesis, The University of Tennessee. Knoxville, TN. 165 pp. Kruger, D., R. H. Petersen & K. W. Hughes (2006). Molecular phylogenies and mating study data in Polyporus with special emphasis on group "Melanopus" (Basidiomycota). Mycological Progress 5: 185-206. Kruger, D. & A. Gargas (2010). Unusual polypore fungi--a taxonomic emendation of Polyporus (Basidiomycotina) after ribosomal spacer characters. Cryptogamie, Mycologie 31: 389–401. Larsen, M. J. & F. F. Lombard (1988). The status of Meripilus giganteus (Aphyllophorales, Polyporaceae) in North America. Mycologia 80: 612–621. Larsen, M. J. & X. Melo (1996). Neotypification of Phellinus pini. Mycologia 88: 839–843. Larsson, K. H., E. Parmasto, M. Fischer, E. Langer, K. K. Nakasone & S. A. Redhead (2006). Hymenochaetales: a molecular phylogeny for the hymenochaetoid clade. Mycologia 98: 926–936. Lowe, J. L. (1975). Polyporaceae of North America. The genus Tyromyces. Mycotaxon 2: 1–82. Medeiros, P. S. & L. Ryvarden (2011). The genus Microporellus Murrill in South America. Synopsis Fungorum 29: 71–73. Miller, S. L., E. Larsson, K. H. Larsson, A. Verbeken & J. Nuytinck (2006). Perspectives in the new Russulales. Mycologia 98: 960–970. Moncalvo, J. M., H. H. Wang & R. S. Hseu (1995). Phylogenetic relationships in Ganoderma inferred from the internal transcribed spacers and 25S ribosomal DNA sequences. Mycologia 87: 223–238. Motato-Vásquez, V. & A. de Mello Gugliotta (2016). The genus Microporellus (Basidiomycota, Polyporales) in the Neotropics. Nova Hedwigia 103: 225–238. Murrill, W. A. (1903). The Polyporaceae of North America—V. The genera Cryptoporus, Piptoporus, Scutiger and Porodiscus. Bulletin of the Torrey Botanical Club 30: 423–434. Murrill, W. A. (1905). The Polyporaceae of North America--XII. A synopsis of the white and bright-colored pileate species. Bulletin of the Torrey Botanical Club 32: 469–493. Murrill, W. A. (1915). Western Polypores. New York: W. A. Murrill. 36 pp. Niemelä, T. & R. Saarenoksa (1989). On Fennoscandian polypores 10. Boletopsis leucomelaena and B. grisea described and illustrated. Karstenia 29: 12–28. Niemelä, T. & O. Miettinen (2008). The identity of Ganoderma applanatum (Basidiomycota). Taxon 57: 963–966. Nunez, M. & L. Ryvarden (1995). Polyporus (Basidiomycotina) and related genera. Oslo, Norway: J. Cramer. 85 pp. Overholts, L. O. (1953). The Polyporaceae of the United States, Alaska and Canada. Ann Arbor: University of Michigan Press. 466 pp. Palacio, M., R. M. B. da Silveira & G. L. Robledo (2019). Neofavolus subpurpurascens comb. nov., with new records from the Neotropics. Phytotaxa 405: 180–186. Palacio, M., E. R. D. dos Santos, N. Menolli Jr. & R. M. B. da Silveria (2021). An overview of Favolus from the Neotropics, including four new species. Mycologia 113: 759–775. Palacios-Pacheco, M. R., T. Raymundo & R. Valenzuela (2010). Primer registro de Ischnoderma resinosum (Schrad.) P. Karst. y Tyromyces fissilis (Berk. & M. A. Curtis) Donk (Polyporales, Basidiomycota) en México. Polibotánica 30: 35–41. Pouzar, Z. (1990). Additional notes on the taxonomy and nomenclature of Ischnoderma (Polyporaceae). Česká Mykologie 44: 92–100. Rizzo, D. M. et al. (2003). Phellinus coronadensis: a new species from southern Arizona, USA. Mycologia 95: 74–79. Ryvarden, L. & T. Iturriaga (2003). Studies in neotropical polypores 10. New polypores from Venezuela. Mycologia 95: 1066–1077. Seelan, J. S. S., A. Justo, L. G. Nagy, E. A. Grand, S. A. Redhead & D. Hibbett (2015). Phylogenetic relationships and morphological evolution in Lentinus, Polyporellus and Neofavolus, emphasizing southeastern Asian taxa. Mycologia 107: 460–474. Shen, L. L., M. Wang, J. L. Zhou, J. H. Xing, B. K. Cui & Y. C. Dai (2019). Taxonomy and phylogeny of Postia. Multi-gene phylogeny and taxonomy of the brown-rot fungi: Postia (Polyporales, Basidiomycota) and related genera. Persoonia 42: 101–126. Shen, Q. et al. (2002). Molecular phylogenetic analysis of Grifola frondosa (maitake) reveals a species partition separating eastern North America and Asian isolates. Mycologia 94: 472–482. Smith, E. C. (1930). Trametes hispida a destructive parasite in apple orchards. Mycologia 22: 221–222. Sotome, K., T. Hattori, Y. Ota, C. To-anun, B. Salleh & M. Kakishima (2008). Phylogenetic relationships of Polyporus and morphologically allied genera. Mycologia 100: 603–615. Sotome, K., Y. Akagi, S. S. Lee, N. K. Ishikawa & T. Hattori (2013). Taxonomic study of Favolus and Neofavolus gen. nov. segregated from Polyporus (Basidiomycota, Polyporales). Fungal Diversity 58: 254–266. Spaulding, P. (1905). A disease of black oaks caused by Polyporus obtusus Berk. Missouri Botanical Garden Annual Report 1905: 109–116. Stalpers, J. A. (1992). Albatrellus and the Heriaceae. Persoonia 14: 537–541. Stalpers, J. A. (1993). The aphyllophoraceous fungi I. Keys to the species of the Thelephorales. Studies in Mycology 35: 1–168. Szczepkowski, A., B. Gierczyk & A. Kujawa (2019). Buglossoporus quercinus, a rare wood-inhabiting fungus on ancient oak trees in Poland: Ecology, distribution and extinction risk assessment. Baltic Forestry 25: 178–186. Tedersoo, L., T. Suvi, K. Beaver & I. Saar (2007). Ectomycorrhizas of Coltricia and Coltriciella (Hymenochaetales, Basidiomycota) on Caesalpiniaceae, Dipterocarpaceae and Myrtaceae in Seychelles. Mycological Progress 6: 101–107. Tomsovsky, M., M. Kolarik, S. Pazoutovz & L. Homolka (2006). Molecular phylogeny of European Trametes (Basidiomycetes, Polyporales) species based on LSU and ITS (nrDNA) sequences. Nova Hedwigia 82: 269–280. Tomsovsky, M. (2012). Delimitation of an almost forgotten species Spongipellis litschaueri (Polyporales, Basidiomycota) and its taxonomic position within the genus. Mycological Progress 11: 415–424. Valenzuela, R., T. Raymundo & J. Cifuentes (2013). El género Inonotus s. l. (Hymenochaetales: Agaricomycetes) en México. Revista Mexicana de Biodiversidad: S70–S90. Vlasák, J., J. Vlasák Jr., P. G. Harvey, P. R. Leacock & V. Spirin (2017). Pyrofomes juniperinus comb. nova, the North American sibling of P. demidoffii (Polyporales, Basidiomycota). Annales Botanici Fennici 55: 1–6. Volk, T. J. (2000). Polypore primer: An introduction to the characters used to identify poroid wood decay fungi. McIlvainea 14: 74–82. Wagner, T. & Fischer, M. (2002). Proceedings towards a natural classification of the worldwide taxa Phellinus s. l. and Inonotus s. l., and phylogenetic relationships of allied genera. Mycologia 94: 998–1016. Watling, R. & J. Milne (2008). The identity of European and North American Boletopsis spp. (Basidiomycota; Thelephorales, Boletopsidaceae). North American Fungi 3: 5–15. Watson, D. M. & D. Shaw (2018). Veiled polypore (Cryptoporus volvatus) as a foraging substrate for the white-headed woodpecker (Picoides albolarvatus). Northwestern Naturalist 99: 58–62. Xing, X., X. Ma, M. M. Hart, A. Wang & S. Guo (2013). Genetic diversity and evolution of Chinese traditional medicinal fungus Polyporus umbellatus (Polyporales, Basidiomycota). PLOS ONE 8 (3): e58807. Zeller, S. M. (1915). Notes on Cryptoporus volvatus. Mycologia 7: 121–125. Zhou, J. -L. & B. -K. Cui (2017). Phylogeny and taxonomy of Favolus (Basidiomycota). Mycologia 109: 766–779. Zhou, L. -W. & 12 coauthors (2016). Polypore diversity in North America with an annotated checklist. Mycological Progress 15: 771–790. This site contains no information about the edibility or toxicity of mushrooms. Cite this page as: Kuo, M. (2022, October). The polypores. Retrieved from the MushroomExpert.Com Web site: http://www.mushroomexpert.com/polypores.html © MushroomExpert.Com |